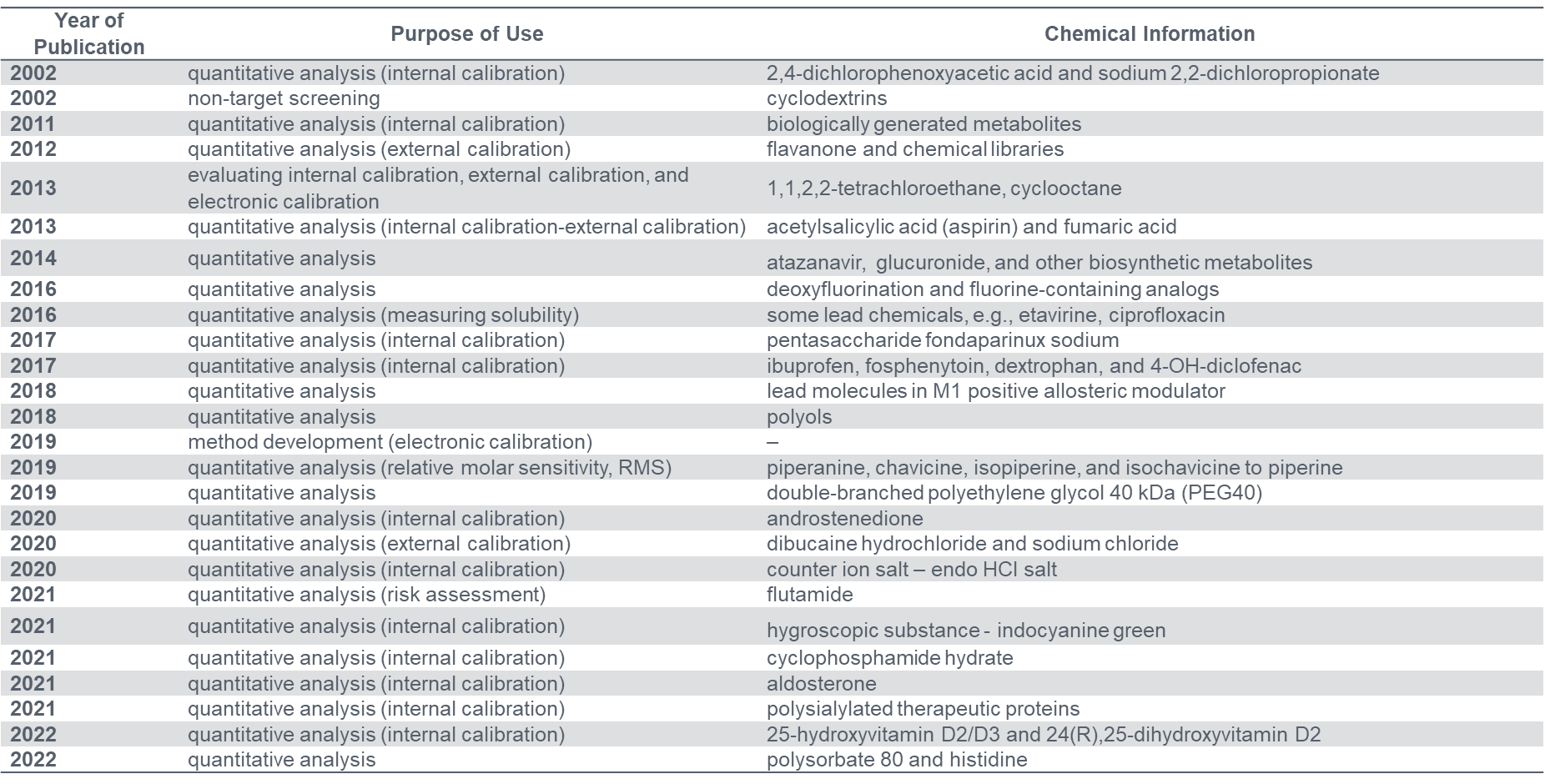
The use of qNMR has been adopted by the pharmaceutical industry in early drug development testing and for testing potency, solvent quantification, residual solvents in drugs, and relative response factor determination. However, it is relatively uncommon that scientists from industries report the details of their methods in literature accessible to the public.
Feedback from the qNMR community indicates that qNMR method development and validation do not contain much novel knowledge that needs to be updated for publication in a peer-reviewed journal. However, as discussed in the section Regulatory Organizations, these organizations and agencies need to acquire more scientific evidence and opinions from qNMR communities in order to approach better practices and decide if qNMR can be the official analytical assay method.
Accordingly, all qNMR practitioners in the pharmaceutical industry are encouraged to publish relevant data as much as possible, with consideration of intellectual property protection where analytical procedures are commercially confidential.
Some qNMR Applications in the Pharmaceutical Industry
Reference/Book Chapter: Quantitative NMR in Qulality Control
in Quality Control of Chinese Medicines-Strategies and Methods